В журнале Journal of the American Chemical Society (JACS), (IF 15,6) опубликована статья с участием сотрудников лаборатории магнитного резонанса Института: м.н.с. А.Е. Райзвиха, к.х.н. Д.В. Трухина (снс), к.х.н. О.Ю. Рогожниковой (снс), к.х.н. В.М. Тормышева (снс, ЛМР) и д.ф.-м.н., проф. Е.Г. Багрянской (Директор НИОХ)
From Silent Precursor to Persistent Reporter: Intracellular Targeting of a Quinone Methide for Triarylmethyl Radicals Accumulation
Arthur E. Raizvikh, Sergey S. Ovcherenko, Olga A. Kartina, Dmitry V. Trukhin, Olga Y. Rogozhnikova, Victor M. Tormyshev, Vladimir V. Koval,Elena G. Bagryanskaya
J. Am. Chem. Soc. 2025, 64, 43, 21772–21780
Publication Date: October 23, 2023
doi: 10.1021/jacs.5c13124
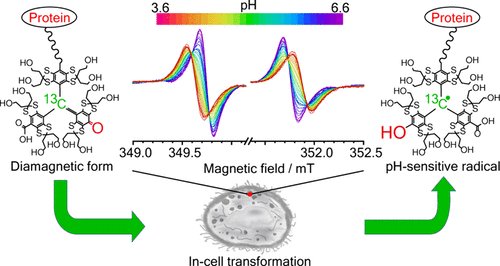
Abstract
Real-time monitoring of critical tumor microenvironment parameters, such as intracellular pH, remains challenging yet essential for understanding cancer progression and therapeutic resistance. We recently observed that the trityl radical OX063 undergoes consecutive oxidation to a diamagnetic quinone methide (QM) under superoxide-generating conditions in culture media with high thiol concentrations. This is followed by thiol-mediated reduction to OX063-OH: a structurally modified, pH-sensitive trityl radical in which one carboxyl group is replaced by a hydroxyl moiety. In this study, we developed a method to target this QM adduct within the intracellular compartment by conjugating it to a cell-penetrating peptide for active transport. Inside cells, the QM adduct converts into the free OX063-OH spin probe. This intracellular probe persists over 10-fold longer than when measured in vivo, where it is rapidly cleared by the kidneys. We demonstrate that this trapped intracellular spin probe effectively reports on physiological properties, including intracellular pH and mobility. It provides a signal strength sufficient for in vivo imaging of these intracellular parameters for over 15 h. This technology has potential applications in metabolic diseases (such as lysosomal storage disorder) as well as studying tumor oxygenation. This redox-activated probe establishes a novel strategy for the noninvasive assessment of tumor acidosis, with significant implications for personalized diagnostics and therapy evaluation.

