На сайте журнала Bioorganic Chemistry (IF 4,7) опубликована статья, соавторами которой являются ученые лаборатории медицинской химии НИОХ СО РАН: к.х.н., нс К.П. Черемных, к.х.н., снс В.А. Савельев и д.х.н., проф. , гнс Э.Э. Шульц. Статья написана в кооперации с учёными из Института молекулярой биологии и биофизики, НГУ и Научно-образовательного центра Н.М.Кижнера Томского политехнического университета.
A versatile synthetic approach to various 5-alkynyl modified isatin derivatives: Cytotoxicity, acetylcholinesterase inhibition activity and molecular modeling study
Kirill P. Cheremnykh, Igor D. Ivanov, Mohammad S. Hamad, Andrey I. Khlebnikov, Victor A. Savelyev, Mikhail A. Pokrovsky, Andrey G. Pokrovsky, Elvira E. Shults
Bioorganic Chemistry, Available online 27 September 2025, 109038
DOI: 10.1016/j.bioorg.2025.109038
Highlights
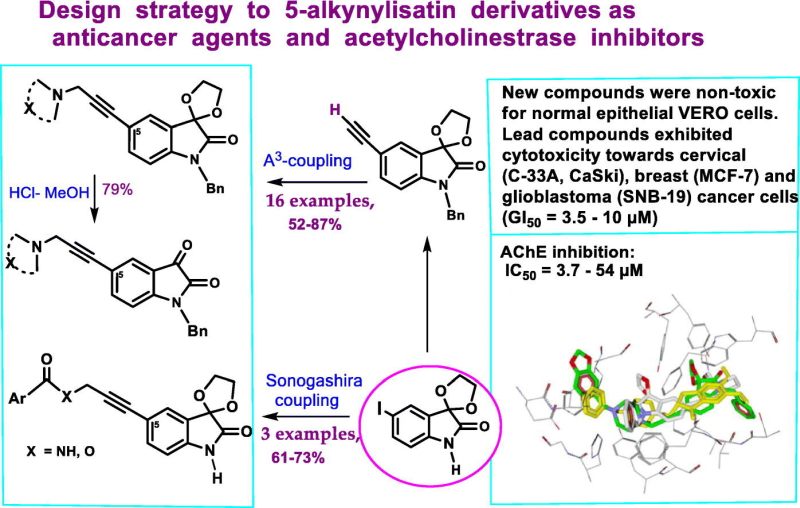
- Synthesis and reactivity of 5-ethynyl derivatives of 3-(1,3-dioxolano)isatins.
- Synthesis of 5-(3-X-propynyl)isatins by A(Chauhan et al., 20213)-coupling reaction of 5-ethynylisatins.
- Excellent yields were obtained by Cu(OAc)2 catalyst, without ligand and additives
- Promising selectivity profiles towards cervical and glioblastoma cancer cells.
- 5-(3-(4-Piperonyl)piperazynyl-propynyl)isatin derivative as AChE inhibition in vitro.
Abstract
Isatin derivatives have been of great interest in drug development research. 5-Alkynyl substituted isatin derivatives with anticancer potential and acetylcholinesterase inhibition (AChE) activity are designed and synthesized. The copper(I) catalyzed one-pot three-component reaction (AChauhan et al. (2021)3-coupling) of new 3-(1,3-dioxolane)-5-(ethynyl)isatins with formaldehyde and secondary amines or the Sonogashira cross-coupling reaction of C-3 protected iodoisatins with fluoro-N-(prop-2-ynyl)benzamide and prop-2-ynyl 4-fluorobenzoate were the main approaches for the synthesis of 5-(3-X-prop-1-yn-1-yl) substituted isatin derivatives (yield 53–87 %). 1-Benzyl-5-(prop-1-yn-1-yl)indoline-2,3-diones are smoothly formed by refluxing of 3-(1,3-dioxolane)-5-(propynyl)isatins in hydrochloric acid/MeOH (1:9, v/v). Results of in vitro biological assays (MTT-test) revealed that new 3-(1,3-dioxolane)-5-(3-X-prop-1-yn-1-yl)-1-benzylisatin derivatives are exhibited remarkable cytotoxicity against human cervical (C 33 A and CaSki), breast (MCF-7), prostate (DU-145) and glioblastoma (SNB-19 and T98G) cancer cell lines within low micromolar GI50 values. Additionally, all new compounds demonstrated relatively low cytotoxicity against the normal epithelial VERO cells (GI50 > 70 μM), indicating good selectivity. Moreover, the appropriate isatin derivatives displayed good and moderate acetylcholinesterase inhibition activity in vitro (Ellman’ s method) with IC50 up to 1.0–3.7 μM comparable to that for clinically used galantamine. Based on the results of in silico experiments the isatin derivatives bearing 5-(prop-1-yn-1-yl) substituents are promising for further search of acetylcholinesterase inhibitors in this series of compounds.
Graphical abstract