В журнале Molecules (Издательство MDPI, IF 4,2) по результатам работы, выполненной совместно с коллегой из Китая ( PetroChina Company Limited, Daqing) опубликована статья, соавторами которой является сотрудники Института: к.х.н. Я.В. Зонов (снс, ЛГС), аспирант В.В. Комаров (мнс, ЛГС), д.х.н. В.М. Карпов (гнс, ЛГС), к.х.н. Д.А. Пархоменко (снс, ЛМР) и д.х.н. Т.В. Меженкова (завлаб ЛГС)
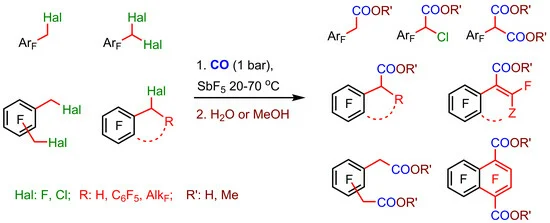
Carbonylation of Polyfluorinated Alkylbenzenes and Benzocycloalkenes at the Benzyl C-F and C-Cl Bonds Under the Action of CO/SbF5
Yaroslav V. Zonov, Siqi Wang, Vladislav V. Komarov , Victor M. Karpov, Dmitriy A. Parkhomenko and Tatyana V. Mezhenkova
Molecules 2025, 30(4), 931;
Published: 17 February 2025
DOI: 10.3390/molecules30040931
(This article belongs to the Section Organic Chemistry)
Abstract