В журналT Inorganic Chemistry Communications (IF 4,4) опубликована статья, соавторами которой являются ученые из ИНХ СО РАН, НИИМББ ФИЦ ФТМ и НИОХ СО РАН (к.х.н. А. М. Агафонцев (снс ЛТС) и д.х.н., проф. А.В. Ткачев (завлаб ЛТС) :
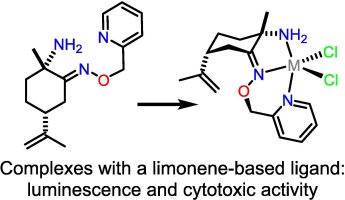
Copper(ii), zinc(ii) and cadmium(ii) complexes with the o-picolyl ether of chiral α-aminooxime of natural (+)-limonene: synthesis, structures, luminescence and cytotoxicity
Tatiana E. Kokina, Alexander M. Agafontsev, Nikita A. Shekhovtsov, Ludmila A. Glinskaya, Kristina D. Sizintseva,
I. Rakhmanova, Yulia A. Golubeva, Lubov S. Klyushova, Alexey V. Tkachev, Mark B. Bushuev
Inorganic Chemistry Communications, V. 170, Part 2, December 2024, 113381
Available online 22 October 2024, , 113381
DOI: 10.1016/j.inoche.2024.113381
Highlights
-
A new chiral α-aminooxime of natural (+)-limonene bearing a pycolyl group (L) was synthesized.
-
Mononuclear complexes, [CuLCl2], [ZnLCl2] and [CdLCl2], were synthesized.
-
The ligand L and the complexes [ZnLCl2] and [CdLCl2] exhibit fluorescence.
-
The complexes [CuLCl2] and [CdLCl2] display cytotoxicity.
Graphical Abstract
Mononuclear complexes with a chiral α-aminooxime of natural (+)-limonene show luminescence and cytotoxic activity.
Abstract
Copper(II), zinc(II) and cadmium(II) complexes, [CuLCl2], [ZnLCl2] and [CdLCl2], with a new derivative of chiral α-aminooxime of natural (+)-limonene bearing a pycolyl group (L) were synthesized. The complexes are mononuclear, the L molecules act as N,N,N-tridentate chelating ligands. The computations support the fact that the N,N,N–chelation by a metal ion is thermodynamically preferred over the N,N–chelation for all complexes. The ligand L and the complexes [ZnLCl2] and [CdLCl2] exhibit fluorescence, with the emission spectra depending on the excitation wavelength. L emits in close to white region, while the Zn(II) and Cd(II) complexes demonstrate blue emission. Due to chelation-induced fluorescence enhancement effect, the fluorescence quantum yield increases when going from L to [ZnLCl2] and [CdLCl2]. The complex [CdLCl2] displays cytotoxicity against the HepG2 cell line (IC50 = 14.3 ± 1.5 μM), which is almost twice as high as that of cisplatin. The human breast adenocarcinoma cell line (MCF-7) is more sensitive to [CuLCl2] (IC50 = 43.4 ± 0.4 μM) than to [CdLCl2].