В журнале ChemPlusChem (IF 3,4) опубликована совместная статья сотрудников НИОХ и ученых из Юго-западного университета Цзяотун, Китай:
Halide Complexes of 5,6-Dicyano-2,1,3-Benzoselenadiazole with 1 : 4 Stoichiometry: Cooperativity between Chalcogen and Hydrogen Bonding
Ekaterina A. Radiush, Prof. Dr. Hui Wang, Dr. Elena A. Chulanova, Yana A. Ponomareva, Bin Li, Qiao Yu Wei, Dr. Georgy E. Salnikov, Svetlana Yu. Petrakova, Dr. Nikolay A. Semenov, Prof. Dr. Andrey V. Zibarev
ChemPhysChem, Volume 88, Issue11, November 2023, e202300523
https://doi.org/10.1002/cplu.202300523
Graphical Abstract
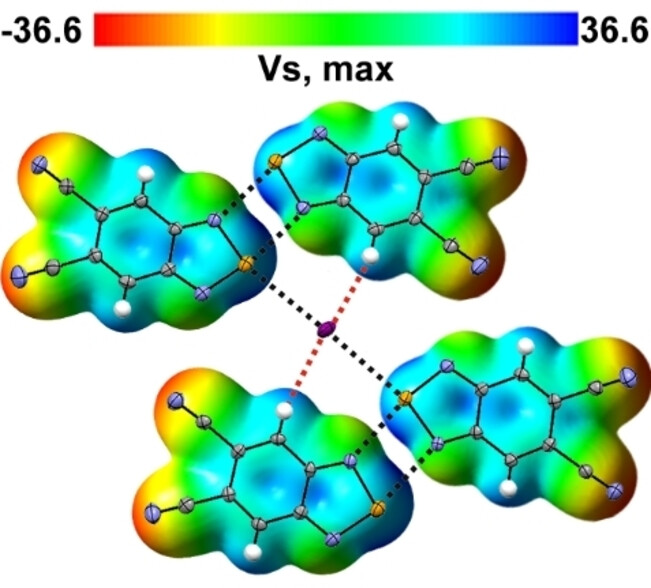
Crystalline complexes of 5,6-dicyano-2,1,3-benzoselenadiazole with [Hal]− (Hal=Cl, Br and I) feature unprecedented 4 : 1 stoichiometry caused by cooperative interplay of chalcogen and hydrogen bondings. Solution UV-Vis spectra of the complexes contain [Hal]−-dependent charge-transfer bands allowing identification of the individual [Hal]−, and the parent selenadiazole can be considered as a chromogenic receptor and prototype sensor of [Hal]−.
Abstract
The [M4−Hal]− (M=the title compound; Hal=Cl, Br, and I) complexes were isolated in the form of salts of [Et4N]+ cation and characterized by XRD, NMR, UV-Vis, DFT, QTAIM, EDD, and EDA. Their stoichiometry is caused by a cooperative interplay of σ-hole-driven chalcogen (ChB) and hydrogen (HB) bondings. In the crystal, [M4−Hal]− are connected by the π-hole-driven ChB; overall, each [Hal]− is six-coordinated. In the ChB, the electrostatic interaction dominates over orbital and dispersion interactions. In UV-Vis spectra of the M+[Hal]− solutions, ChB-typical and [Hal]−-dependent charge-transfer bands are present; they reflect orbital interactions and allow identification of the individual [Hal]−. However, the structural situation in the solutions is not entirely clear. Particularly, the UV-Vis spectra of the solutions are different from the solid-state spectra of the [Et4N]+[M4−Hal]−; very tentatively, species in the solutions are assigned [M−Hal]−. It is supposed that the formation of the [M4−Hal]− proceeds during the crystallization of the [Et4N]+[M4−Hal]−. Overall, M can be considered as a chromogenic receptor and prototype sensor of [Hal]−. The findings are also useful for crystal engineering and supramolecular chemistry.
Альметрики:
Метрики PlumX теперь доступны в Scopus: узнайте, как другие ученые используют ваши исследования