В журнале JOC - The Journal of Organic Chemistry (IF 3,6) опубликована статья с участием сотрудников Института: к.х.н. Д.Г. Мажукина (снс, ЛАС) и д.х.н. А.Я. Тихонова (гнс ЛГетС):
Iodine-Catalyzed Radical C–H Amination of Nonaromatic Imidazole Oxides: Access to Cyclic α-Aminonitrones
Alexey A. Akulov, Mikhail V. Varaksin, Anna A. Nelyubina, Anton N. Tsmokaluk, Dmitrii G. Mazhukin, Alexsei Y. Tikhonov, Valery N. Charushin, and Oleg N. Chupakhin
J. Org. Chem., 2024, 89, 1, 463–473
Publication Date:December 13, 2023
doi: 10.1021/acs.joc.3c02230
Abstract
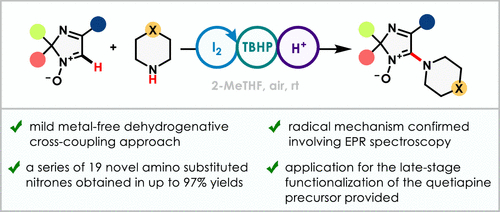
A straightforward cross-dehydrogenative coupling approach to incorporate alicyclic amino residues into the structure of model cyclic aldonitrones, 2H-imidazole oxides, is reported. The elaborated C(sp2)–H functionalization is achieved by employing cyclic amines in the presence of the I2–tert-butyl hydroperoxide (TBHP) reagent system. As a result, a series of 19 novel heterocyclic derivatives were obtained in yields of up to 97%. A mechanistic study involving electron paramagnetic resonance spectroscopic experiments allowed the radical nature of the reaction to be confirmed. In particular, the envisioned mechanistic rationale comprises N-iodination of a cyclic amine, followed by N–I bond homolysis of the resulting intermediate and subsequent amination of the nitrone moiety via the newly generated nitrogen-centered radical.
Альметрики:
Метрики PlumX теперь доступны в Scopus: узнайте, как другие ученые используют ваши исследования