В журнале The Journal of Physical Chemistry A (IF 2,9) опубликована статья, соавтором которой является сотрудник Института д.х.н. А.В. Зибарев (гнс ЛГетС), (с коллегами из Китая - School of Physical Science and Technology, Southwest Jiaotong University, Chengdu 610031, P. R. China). Приглашенная статья в специальном выпуске “Steve Scheiner Festschrift".
Regulation of Secondary Bonding Interactions in Dimers of N‑Methyl-2,1,3-benzochalcogenadiazolium Halides by the Nature of Chalcogens and Halogens and Solvation in Polar Media
Yue Zhang, Qiaoyu Wei, Hui Wang, Hongyan Wang, Andrey V. Zibarev
J. Phys. Chem. A 2025, 129, 38, 8846–8857,
Published as part of The Journal of Physical Chemistry A special issue “Steve Scheiner Festschrift.”
Publication Date (Web):September 14, 2025
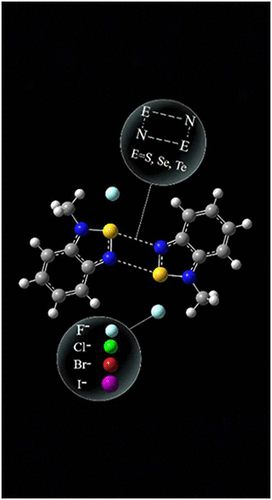
Graphical Abstract
AbstractN-Methylation of 2,1,3-benzochalcogenadiazoles transforms them into the title [RE]+ cations (E = S, Se, Te) and enhances the capability to secondary bonding interactions (SBIs). In the gas phase and CH2Cl2 solvent, [RE]+ can form dimeric ([RE]+[X]−)2 (X = F, Cl, Br, I) complexes by means of E···N and E···X SBIs regulated by the nature of E and X, as well as by solvation effects. The E···X SBI prevails in the gas phase, while the E···N SBI prevails in CH2Cl2. QTAIM confirms stronger solvated E···N SBI for heavier E. SAPT energy decomposition suggests electrostatics as the primary driver of the E···X SBI, with contributions increasing in CH2Cl2 with E polarizability, but decreasing with larger van der Waals bulk of [X]−. Charge transfer (CT) disclosed for the E···N and E···X SBIs with QTAIM and NBO analyses suggests their partial covalency, particularly via negative hyperconjugation. The second-order stabilization energies are higher with heavier E and bulkier [X]−. TD-DFT-calculated UV–vis spectra of ([RE]+[X]−)2 exhibit CT bands depending on E and solvation effects. These insights are useful for the molecular design of anion-responsive materials.