В журнале Archives of Biochemistry and Biophysics (IF 3,8) по результатам работы, выполненной совместно с коллегами из Института химической кинетики и горения СО РАН и Институт химии твёрдого тела и механохимии СО РАН, опубликована статья, соавторами которой являются сотрудники Института кхн В.В. Фоменко (нс ЛФАВ) и и чл.корр. РАН, д.х.н., проф. Н.Ф. Салахутдинова (зав. отделом ОМХ).
The mutual lipid-mediated effect of the transmembrane domain of SARS-CoV-2 E-protein and glycyrrhizin nicotinate derivatives on the localization in the lipid bilayer
Polina A. Kononova, Olga Yu Selyutina, Vladislav V. Fomenko, Nariman F. Salakhutdinov, Nikolay E. Polyakov
Archives of Biochemistry and Biophysics Volume 758 , August 2024, 110080DOI: https://doi.org/10.1016/j.abb.2024.110080
Highlights
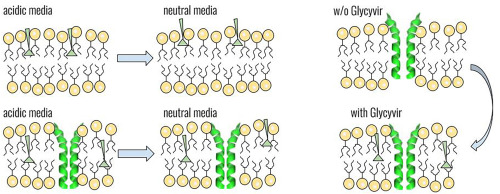
Glycyrrhizin nicotinate (Glycyvir) is anchored in a lipid bilayer in the acidic and neutral media.
Transmembrane domain of SARS-CoV-2 E-protein (ETM) induces deeper penetration of Glycyvir into a lipid membrane.
The presence of Glycyvir leads to deeper immersion of ETM in lipid bilayer.
Abstract
Glycyrrhizinic acid (GA) is one of the active substances in licorice root. It exhibits antiviral activity against various enveloped viruses, for example, SARS-CoV-2. GA derivatives are promising biologically active compounds from perspective of developing broad-spectrum antiviral agents. Given that GA nicotinate derivatives (Glycyvir) demonstrate activity against various DNA- and RNA-viruses, a search for a possible mechanism of action of these compounds is required. In the present paper, the interaction of Glycyvir with the transmembrane domain of the SARS-CoV-2 E-protein (ETM) in a model lipid membrane was investigated by NMR spectroscopy and molecular dynamics simulation. The lipid-mediated influence on localization of the SARS-CoV-2 E-protein by Glycyvir was observed. The presence of Glycyvir leads to deeper immersion of the ETM in lipid bilayer. Taking into account that E-protein plays a significant role in virus production and takes part in virion assembly and budding, the data on the effect of potential antiviral agents on ETM localization and structure in the lipid environment may provide a basis for further studies of potential coronavirus E-protein inhibitors.
GraphicalAbstract