В журнале JOC - The Journal of Organic Chemistry (IF 3,3) опубликована статья, соавторами каторой являются сотрудники лаборатории физиологически активных веществ к.х.н., снс И.В. Ильина, к.х.н., нс О.С. Патрущева, чл.корр. РАН, д.х.н., проф. Н.Ф. Салахутдинова (зав. отделом ОМХ) , д.х.н., проф. РАН, гнс К.П. Волчо и д.х.н. Ю.В. Гатилов (внс, ГРСА):
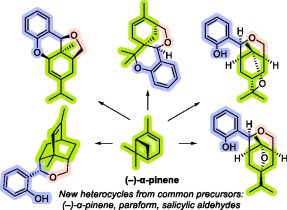
Unusual Cascade Reactions of 8‑Acetoxy-6-hydroxymethyllimonene with Salicylic Aldehydes: Diverse Oxygen Heterocycles from Common Precursors
Irina V. Ilyina, Oksana S. Patrusheva, Victoria V. Goltsova, Kimberley M. Christopher, Yuri V. Gatilov, Alexander Yu Sidorenko, Vladimir E. Agabekov, Nariman F. Salakhutdinov, Igor V. Alabugin , Konstantin P. Volcho
J. Org. Chem., 2024, 2024, V. 89, N 16, 11593-11606
Published on 16 August 2024
doi: 10.1021/acs.joc.4c01282
Abstract
Chiral oxygen-containing heterocyclic compounds are of great interest for the development of pharmaceuticals. Monoterpenes and their derivatives are naturally abundant precursors of novel synthetic chiral oxygen-containing heterocyclic compounds. In this study, acid catalyzed reactions of salicylic aldehydes with (−)-8-acetoxy-6-hydroxymethyllimonene, readily accessible from α-pinene, leads to the formation of chiral polycyclic products of various structural types. Three of the six isolated chiral heterocyclic products obtained from salicylic aldehyde contain previously unknown polycyclic ring types. Having carried out the reaction in the presence of Brønsted or Lewis acids (Amberlyst 15, trifluoromethanesulfonic acid, trifluoroacetic acid and boron trifluoride etherate) or aluminosilicates (montmorillonite K10, halloysite nanotubes), we found that the nature of products depends on the catalyst as well as the reaction conditions (reaction time, reactant ratio, presence or absence of solvent). Detailed mechanistic insight on the complex cascade reactions for product formation is provided with extensive experimental and quantum mechanical computational studies.