В журнале Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, (IF 4.6) опубликована статья, соавторами которой являются сотрудники Лаборатории гетероциклических соединений НИОХ СО РАН: к.х.н., снс Т.А. Ваганова и д.х.н., гнс Е.В. Малыхин:
What makes molecular iodine solid? Not London dispersion forces, but halogen bonds
Haiyan Fanc, Tamara Vaganova, Evgenij Malykhin, Enrico Benassi
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Volume 361, 5 February 2026, 126862
Available online 16 July 2022
doi: 10.1016/j.saa.2025.126862
Highlights
- Halogen bonds (XB) dominate in solid iodine, not London dispersion forces.
- I2 pair showing strongest XB indicates charge transfer
- Raman spectra confirm XB by weakening of the I--I bonds.
- Textbook descriptions of I2 solid-state interactions urge revision
Graphical abstract
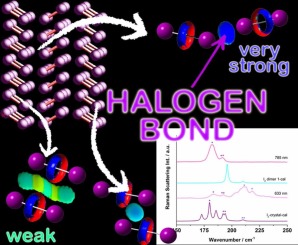
Abstract
The nature of intermolecular interactions in iodine (I₂) crystals has long been a subject of interest, traditionally attributed to London dispersion (LD) forces. Herein, we systematically investigate I₂⋯I₂ interactions through quantum mechanical calculations combined with spectroscopic and theoretical analyses. Focusing on three I₂⋯I₂ dimers extracted from the crystal structure, the nature of these interactions was further clarified using electron localization function (ELF) analysis and topological atoms-in-molecules (AIM). The results confirm XB is dominated in dimer 1, the most stable dimer. Comparisons of experimental Raman spectra with calculated spectra for dimer 1 and crystal fragments revealed frequency splitting of I---I stretching modes, directly linked to XB-induced weakening of specific I---I bonds. Collectively, these results provide the first systematic evidence that the intrinsic intermolecular interaction in I₂ crystals is halogen bonding, rather than LD forces. This work redefines our understanding of iodine's solid-state structure and the nature of the intermolecular interactions.
Альметрики:
Метрики PlumX теперь доступны в Scopus: узнайте, как другие ученые используют ваши исследования

