На сайте журнала Physical Chemistry Chemical Physics, (IF 3,3) опубликована статья с участием сотрудников Института - д.ф.-м.н. , проф. Е.Г. Багрянской (директор Института), к.ф.-м.н. Д.А. Пархоменко (снс, ЛМР) и к.х.н. Д.А. Морозова (зам. директора по науке, снс ЛАС).
A novel method of alkoxyamine homolysis activation via photochemical rearrangement
Sergey Cherkasov, Dmitriy Parkhomenko, Denis Morozovb and Elena Bagryanskaya
Phys. Chem. Chem. Phys.,
2024, 26(12), 9754-9762
doi: 10.1039/D3CP05815H
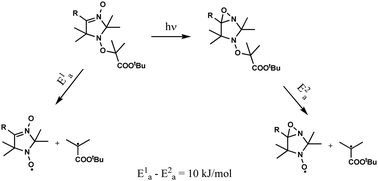
Abstract
We proposed the nitrone–oxaziridine rearrangement as a novel method for photochemical activation for the homolysis of alkoxyamine in nitroxide-mediated polymerization. The photoisomerization of the aldo-/ketonitrone-group into the oxaziridine one in 2,5-dihydroimidazole 3-oxide-based alkoxyamines was studied; the products of photolysis have been identified, and quantum yields were measured. Conversion of the nitrone group into the oxaziridine one was found to decrease the activation energy of alkoxyamine homolysis by ca. 10 kJ mol−1.
Альметрики:
Метрики PlumX теперь доступны в Scopus: узнайте, как другие ученые используют ваши исследования

